The increasing number of antibiotic resistant bacterial strains determines importance of synthesis of new antibacterial compounds and studying of their effects [1]. Organic compounds of selenium are considered as perspective. Recently was shown high antibacterial activity of some selenorganic compounds [3, 5].
At present selenorganic compound 1,5-diphenyl-3-selenapentadion-1,5 or diacetophenonylselenide (DAPS-25) is used in animal and poultry farming in a number of regions of Russia. Synthesis and studying of biological activity of its derivatives are carried out [4].
The aim of the study: to analyze comparative antibacterial activity of selenorganic compound diacetophenonylselenide (DAPS-25) and its chloride-, fluoride- and nitro-derivatives on clinical strains of Escherichia coli extracted from patients with suppurative complications of traumatology-orthopedic hospital.
Materials and methods of research
In this work we used selenorganic compounds 1,5-diphenyl-3-selenapentadion-1,5 (DAPS-25 – compound 1), 1,5-di – (m-nitrophenyl)-3-selenapentadion-1,5 (compound 2), 1,5-di – (p-chlorphenyl)-3-selenapentadion-1,5 (compound 3) and 1,5-di – (p-fluorphenyl)-3-selenapentadion-1,5 (compound 4), kindly given by a professor B.I. Drevko (Figure).
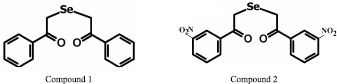
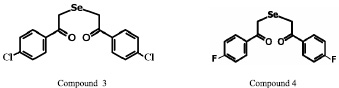
Structures of selenorganic compounds: 1,5-diphenyl-3-selenapentadion-1,5 (compound 1), 1,5-di – (m-nitrophenyl)-3-selenapentadion-1,5 (compound 2), 1,5-di – (p-chlorphenyl)-3-selenapentadion-1,5 (compound 3) and 1,5-di – (p-fluorphenyl)-3-selenapentadion-1,5 (compound 4)
Experiment was carried out on 10 taxonomic identical clinical strains of Escherichia coli (E. coli) extracted from patients with suppurative complications which are on treatment in a traumatology and orthopedic hospital of the Saratov scientific research institute of traumatology and orthopedics. Generic identification of strains has carried out on the basis of studying phene. Bacteria had resistance to five and more structural antibiotics. Suspension of bacteria prepared with use the turbidity standard of the State scientific research institute of standardization and the control of medical biological preparations n.a. L.A. Tarasevich, by consecutive cultivations to final concentration of bacteria – 3·105 cells in 1 ml.
For investigation of antibacterial action we prepared 4 dilutions of selenorganic compounds in concentrations 0,001–1 mg/ml. The mix of dimethylformamide (DMFA) in 0,9 % solution NaCl in the relation 1:10 is used as a solvent. Aliquot of 100 μl of final suspension of microorganisms was added in test tubes with diluted compounds and incubated for 90 minutes at a room temperature. As the control group used the same quantities of bacterial suspension dissolved in similar proportions with the solvent (DMFA in 0,9 % solution NaCl) and incubated for the same time interval. Then aliquot of 100 μl of bacterial suspensions from each test tube inoculated and spread on nutrient meat-peptonic agar which was incubated for 24 hours at 37 °С. Counting of colonies was made next day.
Statistical analysis of finding carried out by means of software package Statistica 6.0. We checked hypotheses about a kind of distributions (Shapiro-Wilk’s criterion). A lot of findings did not fit of distribution law. For comparison of values the U-Mann-Whitney’s criterion, Z – Fisher’s criterion and a p-value were determined. A critical significance of p-value in this research accepted equal 0,05.
Results of research and their discussion
The results depicted in Table show the increase of antibacterial activity of selenorganic compounds against clinical strains of E. coli in a direction: 1 → 2 → 3 → 4.
90-minutes incubation of E. coli with selenorganic compounds in maximal concentration 1 mg/ml led to inhibition of bacterial colonies growth on 43,4 % (compound 1), 95,2 % (compound 2), 90,0 % (compound 3) and 100 % (compound 4) correspondingly in comparison with control. Use of selenorganic compounds 2, 3 and 4 in concentration 0,1 mg/ml decreased the quantity of colonies on nutrient agar on 85,1 %, on 51,1 % and on 100 % correspondingly, and in concentration 0,01 mg/ml – on 64,8; 31,2 and 94,6 % correspondingly in the comparison with control. Among the studied substances only compounds 3 and 4 in minimal concentration 0,001 mg/ml significantly reduced growth of E. coli colonies on 29,3 and 77.6 % correspondingly.
Antibacterial effect of selenorganic compounds 1–4 on clinical strains of E. coli
|
Compounds |
The quantity of colonies on nutrient agar |
||||
|
Control group |
Experimental groups, concentration of compound, mg/ml |
||||
|
1 |
0,1 |
0,01 |
0,001 |
||
|
1 |
821 (671; 907) |
465 (256; 569) Zк = 2,72; pк = 0,006502, |
685 (585; 1045) Zк = 0,30; pк = 0,762369, |
929 (759; 1063) Zк = 1,47; pк = 0,140466, |
762 (563; 983) Zк = 0,04; pк = 0,969850, |
|
2 |
1046 (848; 1155) |
50 (9; 163) Zк = 3,77; pк = 0,000157, |
156 (94; 224) Zк = 3,77; pк = 0,000157, |
368 (241; 490) Zк = 3,77; pк = 0,000157, |
977 (790; 1093) Zк = 1,13; pк = 0,256840, |
|
3 |
982 (867; 1108) |
98 (74; 207) Zк = 3,77; pк = 0,000157, |
480 (231; 652) Zк = 3,55; pк = 0,000381, |
676 (561; 786) Zк = 3,32; pк = 0,000881, |
694 (456; 841) Zк = 2,72; pк = 0,006502, |
|
4 |
1003 (895; 1089) |
0 (0; 6) Zк = 3,77; pк = 0,000157, |
1 (0; 9) Zк = 3,77; pк = 0,000157, |
54 (11; 118) Zк = 3,77; pк = 0,000157, |
225 (67; 342) Zк = 3,77; pк = 0,000157, |
Notes: In each case is given median value, lower and top quartiles (25; 75 %). Zк, pк – differences in comparison with control group.
Findings allow suggesting the part of lateral groups at the phenyl radicals in antibacterial effect of compounds 2, 3 and 4. For example, compound 1, lost of lateral groups at the phenyl radicals demonstrated minimal antibacterial effect on E. coli. Compound 2 has two nitro-groups in meta-position of phenyl radicals. It allows drawing an analogy with antibacterial effect of nitrofurans. It is known that nitrofurans get antibacterial activity after reduction of nitro-groups by flavin-dependent nitro-reductases. They localize in bacteria, protozoa and tissues of organism. Intermediate products of consistent one-or two-electronic stages of reduction are highly reactive, especially nitro-radical anion due to nitrofurans get antibacterial activity [2].
Besides compounds 3 and 4 have chlorine and fluorine atoms in para-position of phenyl rings. It is known that fluorine is the most electronegative element and the most powerful oxidizer. Due to presence of two fluorine atoms the compound 4 was got prooxidant properties and as consequence was become the most toxic of all investigated selenorganic compounds showing the maximal antibacterial activity. Oxidizing properties of chlorine are much weaker, than at fluorine. Therefore the chlorine-containing analogue of DAPS-25 (compound 3) has smaller antimicrobic activity in comparison with compound 4.
In this connection, it is possible to conclude that antibacterial activity of selenorganic compounds is caused by presence/absence of lateral groups at the phenyl radicals in its structure. Due to lateral groups the compounds have got toxicity and have had an antibacterial effect.
Conclusion
Findings allow suggesting the perspectives of using of selenorganic compounds 1,5-di – (m-nitrophenyl)-3-selenapentadion-1,5 (compound 2), 1,5-di – (p-chlorphenyl)-3-selenapentadion-1,5 (compound 3) and 1,5-di – (p-fluorphenyl)-3-selenapentadion-1,5 (compound 4) as antibacterial compound against antibiotic-resistant bacterial strains of E. coli.

